REGULATION MECHANISMS FOR COLD STRESS RESPONSES OF FISH
-
摘要: 鱼类遭受低温胁迫易产生分子、细胞和组织损伤, 甚至导致个体死亡。鱼类机体细胞感受到低温刺激后, 通过多种应激通路将低温信号传递至细胞核, 启动低温应激反应, 建立新的胞内稳态, 从而增强抗寒能力。低温可激活鱼类内分泌系统释放皮质醇和甲状腺素等激素, 调控代谢、渗透压和免疫反应, 最终引起生理和行为变化。鱼类低温应激反应受表观遗传修饰、转录和翻译、前体RNA可变剪接和蛋白质翻译后修饰等多层级的复杂调控。目前, 采用多组学技术已经鉴定到大量与低温响应相关的效应基因和调控通路。研究表明, 能量代谢和抗氧化应激反应在鱼类抗寒能力的建成起着重要作用。其他环境因子(如低氧和盐度)及鱼体生理状态(如饥饿和营养)也影响鱼类对低温刺激的反应和抗寒能力。鉴定抗寒相关的分子标记并解析其关联基因的作用机制对鱼类的抗寒育种具有重要意义。Abstract: Exposure of fish to hypothermia stress may lead to damage to biological molecules, cells and tissues, and even death. When fish cells sense the cold stress, the cold signals are transduced into the nuclear by a variety of routes to trigger the cold stress responses. A new homeostasis will be established and the resistance of the cells to cold stress will be enhanced through these finely regulated stress responses. The endocrinology system of fish can be activated by cold stress to release hormones such as cortisol and thyroid. These hormones regulate metabolism, osmolarity and immune responses of the body and ultimately lead to alterations in physiology and behavior. Cold stress responses of fish are complexly regulated at multiple levels, including epigenetics, transcription, translation, alternative splicing of pre-mRNAs and post-translational modification of proteins. Recent omics studies have identified many cold responsive genes and metabolism pathways. Energy generation and anti-oxidation responses are critical for the establishment of fish cold resistance. Environmental factors including hypoxia and salinity, and the physiology of the fish such as fasting and nutritious status also can affect the responses and resistance of fish to cold stress. The identification of cold resistance-associated biomarkers and the discovery of functional mechanisms of the linked genes have paramount significance for the breeding of cold-resistant fish strains.
-
Keywords:
- Fish /
- Hypothermia /
- Stress response /
- Cold resistance /
- Genetics and breeding
-
鱼类的抗寒能力往往受遗传、发育阶段和热经历(Thermal history)等因素的综合影响[1, 2]。研究发现, 重要经济鱼类如罗非鱼(Oreochromis sp.)、大黄鱼(Nibea albiflora)[3]、斜带石斑鱼(Epinephelus coioides)[4]和金头鲷(Larimichthys crocea)[5]等均具有不耐寒的特点, 当寒潮来临时经常被大量冻死, 或因免疫力降低而导致大面积病害发生, 给水产养殖业造成巨大的损失。鱼类在长期演化过程中形成了应对低温胁迫的生理、生化和遗传机制, 因此, 可以通过解析对低温应激反应和抗寒起关键作用的遗传因子, 来研发增强鱼类抗寒能力的技术方法。本文主要综述了近年来鱼类低温应激反应与调控, 及抗寒性状遗传决定基础方面的研究进展, 为鱼类生理和遗传育种研究提供参考。
1. 低温胁迫造成分子、细胞和组织损伤
鱼类遭遇急性低温胁迫会产生分子、细胞和组织等多个水平的损伤作用[6]。温度对核酸和蛋白质等细胞组分的结构和功能具有决定性影响[7]。在生理温度条件下, 细胞表达与其环境温度相应的酶和结构蛋白, 形成有利于实现各项生物学功能的细胞内稳态。当鱼体遭遇低温胁迫时, 核酸和蛋白质等生物分子不能正确折叠和组装, 导致其活性降低[8]。低温胁迫还能诱导活性氧(ROS)的产生, 对DNA和蛋白质造成氧化性损伤[6, 9, 10]。
在细胞水平, 低温刺激可降低生物膜(包括细胞膜和细胞器膜)的流动性, 改变膜的结构, 影响膜及膜结合蛋白的功能[11, 12]。在低温条件下, 产生能量的速率降低, 导致细胞能量供应不足[13]。低温影响微管蛋白的多聚化, 降低其稳定性, 从而破坏细胞骨架, 改变细胞形态[14]。另外, 低温能诱导线粒体的超极化并使溶酶体膜的通透性升高[14]。低温暴露还能引起脂肪的过氧化, 激活铁死亡(Ferroptosis)等细胞死亡通路[9, 15]。
在组织水平, 低温暴露可降低心脏的收缩功能, 导致组织缺血, 进而减少组织和细胞的氧气供应, 造成组织缺氧[6]。例如将鲤(Cyprinus carpio)从25℃直接转移到15℃, 暴露90s后其脑部供血量即急剧降低[16], 从而影响神经系统的功能。在低温胁迫条件下, 鱼类鳃的呼吸和渗透压调节功能受抑制[17], 离子平衡被扰乱[1]。低温胁迫还降低鱼体的免疫力[18, 19]、组织的代谢率[20]及游泳和逃避捕食者的能力, 使其被捕食的风险增加[21]。无论在自然还是在养殖条件下, 冬季都会出现鱼类大量死亡的事件, 其主要原因包括低温应激、饥饿和疾病等[22], 这些都与低温对鱼体造成的不利影响有关。
2. 鱼类的低温应激反应及其调控机制
2.1 低温信号的感知和传递
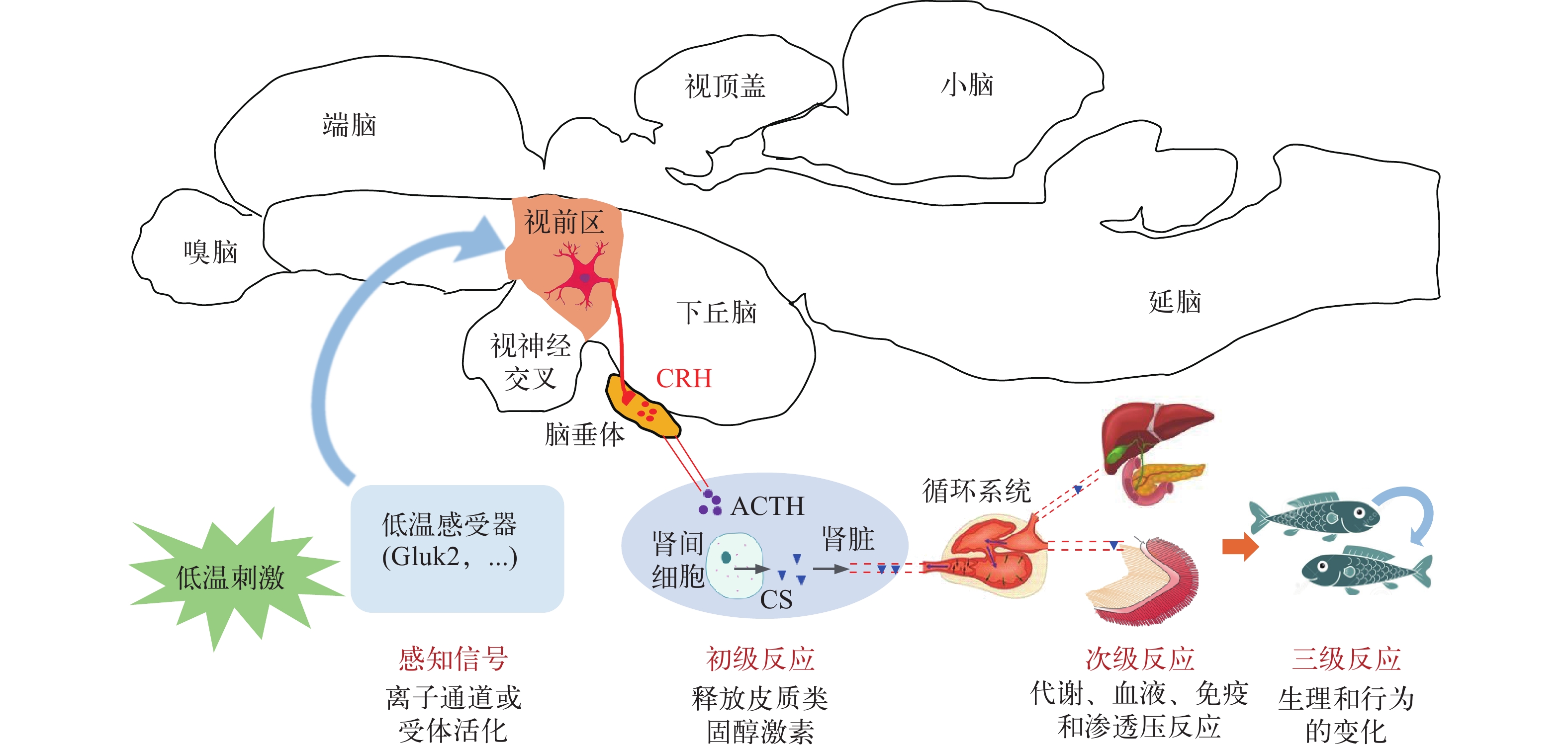
鱼体通过对低温刺激产生应激反应, 建立新的生理、生化和代谢稳态来增强抗寒能力, 这一过程称为“低温适应(Cold acclimation)”。机体和细胞必须感受到低温刺激, 并将刺激信号传递至细胞核才能启动低温应激反应。在低温信号的感知方面, 细菌细胞利用mRNA分子中特定的二级结构作为温度感受器, 控制转录本在不同温度条件下的翻译效率[23]。真核细胞对低温信号的传递主要依赖钙离子信号系统, 低温胁迫激发细胞外钙离子的内流, 进而激活相关的蛋白激酶和转录因子[24, 25]。离体培养的昆虫组织利用钙离子信号感知低温刺激和激活下游的“快速低温强化(Rapid cold hardening, RCH)”机制, 使用特异性抑制剂抑制钙离子的内流、钙调蛋白的激活和钙调蛋白依赖激酶Ⅱ(CaMKII)的活性都能消除RCH的抗寒效应[24]。脊椎动物下丘脑的视前区是控制温度感知和体温调节的区域, 其接收来自外周温度感受器的信号, 调控各种生理和行为的热调节反应(图 1)[26]。瞬时受体电位离子通道TRPM8是在哺乳动物中发现的低温感受器, 其在感觉神经元中特异性表达, 低温刺激时能迅速开放, 介导钙离子的内流[25]。Trpm8敲除的小鼠仍然能感受低温刺激, 说明动物细胞中还存在其他的低温感受器[27]。有研究发现, 在小鼠(Mus musculus)下丘脑中表达的环核苷酸门控离子通道CNGA3也是一个低温感受器[28]。
鱼类的中枢神经系统也参与对低温刺激的感知和低温信号的传递。鲤下丘脑的视前区在受到降温刺激后30s被激活, 其周围的内分泌神经元被激活后可以释放促肾上腺皮质激素释放激素(CRH), 诱导下游的生理反应[16]。通过遗传筛选发现线虫(Caenorhabditis elegans)的GLR-3基因具有低温感受器的功能, 该基因编码一个谷氨酸受体, 其在斑马鱼(Danio rerio)中的同源基因gluk2也具有传递低温信号的功能[29]。离体培养的组织和细胞也能感受低温刺激、传递低温信号和启动低温应激反应[24, 30], 说明还存在不依赖神经系统和细胞自主的低温感受器。鱼类的低温感受器还需进一步鉴定, 钙离子信号系统在鱼类低温信号传递中的作用尚待深入研究。
2.2 内分泌系统在调控低温应激反应中的作用
在受到外界刺激时, 鱼类的下丘脑-垂体-肾间腺(Hypothalamus-pituitary-interrenal, HPI)轴被激活。首先, 由下丘脑释放CRH激活垂体的促皮质细胞, 使其释放促肾上腺皮质激素(ACTH); ACTH诱导肾间细胞释放皮质类固醇激素(CS), 即初级反应; 皮质类固醇激素进入血液系统后调控代谢、血液、渗透压和免疫反应(次级反应), 进而引起个体生理和行为的改变(三级反应, 图 1)[1, 16, 31, 32]。
皮质醇是硬骨鱼类主要的皮质类固醇激素。低温暴露能迅速诱导鱼类血浆中皮质醇含量上升[9, 32]。例如, 将水温在30min内从25℃降低到12℃显著升高了奥利亚罗非鱼(Oreochromis aureus)血浆中皮质醇的含量[33]。血浆中皮质醇的含量与降温的幅度成正相关[34], 因此常被用作鱼体受刺激程度的指标[31]。皮质醇进入细胞后与细胞质中的糖皮质激素受体(GR)结合。GR与激素结合后被激活, 发挥转录因子的功能, 调控糖皮质激素反应基因的表达[32]。皮质醇在调控鱼类的中间代谢、渗透压和免疫中具有重要的作用[32]。低温刺激后奥利亚罗非鱼血浆皮质醇水平的升高与吞噬细胞的活性被抑制有关[33]。皮质醇在鱼类应激反应中的主要作用可能是调控能量的再分配, 促进糖异生以增加细胞能量供应; 同时抑制生长和免疫等耗能的生物学过程, 从而增强细胞抵抗环境胁迫的能力[35]。目前, 皮质醇等糖皮质激素在调控鱼类低温应激反应和抗寒能力形成中的作用和机制还不清楚。
甲状腺素也参与调控鱼类的低温适应。斑马鱼在18℃低温适应的同时, 用丙基硫尿嘧啶(PTU)处理抑制甲状腺素的合成, 能影响能量代谢相关基因的表达和降低低温适应对个体持续游泳能力的增强作用[36]。抑制甲状腺素的合成使个体持续游泳的能力降低, 主要表现为尾部摆动频率下降, 这可能与肌肉组织中肌浆网钙离子ATP酶(SERCA)活性降低和肌球蛋白重链的表达水平下降有关[37]。甲状腺素在斑马鱼低温适应过程中还能通过影响心率和SERCA的活性来增强心脏的功能[38]。
2.3 低温诱导的转录反应及其调控机制
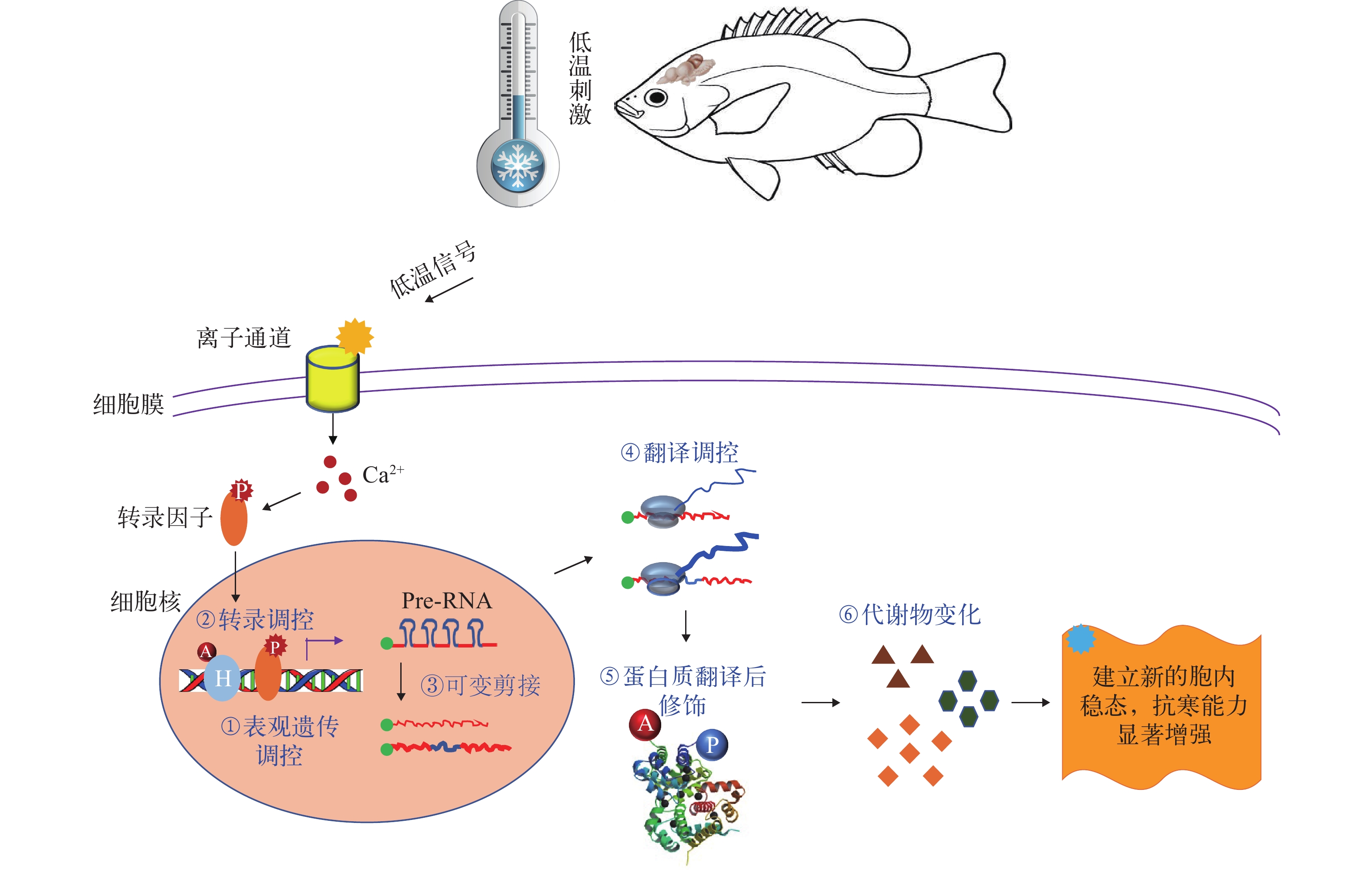
细胞接收到低温信号以后, 通过对基因表达进行精细的调控, 从而建立新的胞内稳态, 修复低温应激造成的分子损伤, 清除受损严重的细胞, 增强机体的抗寒能力。在低温胁迫时, 鱼类的中枢神经系统感受低温刺激。作为低温感受器的离子通道被激活, 使钙离子进入细胞内。胞内游离钙离子浓度升高, 激活相关的激酶, 进而使特定的转录因子发生磷酸化修饰而活化; 活化的转录因子进入细胞核激活下游基因的转录, 完成低温信号的传递(图 2)。
![]() 图 2 鱼类低温应激反应的遗传调控H. 组蛋白; A. 乙酰化修饰; P. 磷酸化修饰; 可变剪接. 绿色的点表示mRNA的5′端帽子结构, 线条中蓝色部分表示内含子, 红色表示外显子; 线条的粗细表示RNA分子的丰度; 翻译调控. 蓝色线条的粗细表示肽链的翻译效率; 代谢物变化. 图形的数目表示不同代谢物的丰度Figure 2. Genetic regulation underlying cold stress responses of fishH. histone; A. acetylation; P. phosphorylation; Alternative splicing: the green dots stand for 5′ cap of mRNA molecules, the blue segment in the lines stands for intron and the red segment stands for exon, while thickness of the lines denotes abundance of the RNA molecules; Translation regulation: thickness of the blue lines indicates translational efficiency of the peptides; Change in metabolites: numbers of the geometric figures represent abundance of different metabolites
图 2 鱼类低温应激反应的遗传调控H. 组蛋白; A. 乙酰化修饰; P. 磷酸化修饰; 可变剪接. 绿色的点表示mRNA的5′端帽子结构, 线条中蓝色部分表示内含子, 红色表示外显子; 线条的粗细表示RNA分子的丰度; 翻译调控. 蓝色线条的粗细表示肽链的翻译效率; 代谢物变化. 图形的数目表示不同代谢物的丰度Figure 2. Genetic regulation underlying cold stress responses of fishH. histone; A. acetylation; P. phosphorylation; Alternative splicing: the green dots stand for 5′ cap of mRNA molecules, the blue segment in the lines stands for intron and the red segment stands for exon, while thickness of the lines denotes abundance of the RNA molecules; Translation regulation: thickness of the blue lines indicates translational efficiency of the peptides; Change in metabolites: numbers of the geometric figures represent abundance of different metabolites转录组研究是揭示鱼类应激反应及其调控机制的重要手段。近年来, 应用基因芯片、RNA-seq和small RNA-seq等技术对多种鱼类进行了研究, 鉴定低温调控的基因、miRNA、lncRNA和转录本的可变剪接事件。研究的种类既有模式鱼, 也有经济鱼类, 既有暖水性鱼类, 也有冷水性鱼类; 低温处理方式有急性暴露, 也有慢性暴露; 刺激程度有温和的、非致死的处理, 也有致死低温暴露; 样品的组织来源也多种多样。虽然不同种类对低温刺激的反应具有很大差异, 但鉴定到了低温应激反应的标记基因, 如低温诱导的RNA结合蛋白cirbp、高迁移率蛋白家族(hmgb)成员和硬脂酰辅酶A去饱和酶(scd)等, 这些基因在大多数情况下都能被低温刺激诱导表达[39-42]。通过对低温响应基因(Cold-responsive gene, CRG)进行基因本体(Gene ontology, GO)和信号通路富集分析发现, RNA剪接、转录调控、生物钟节律和蛋白质分解代谢等是最具代表性的受低温调控的生物学过程[18, 39-41, 43-45], FoxO信号通路在调控鱼类抗寒能力的形成中具有重要作用[46]。不同组织对低温刺激的反应既有共同的特征, 又有很高的组织特异性[39, 44, 45]。特定组织的低温应激反应与其功能相关, 例如肝脏的CRG主要参与能量和脂肪酸代谢[4, 5, 47], 肌肉的CRG主要参与能量代谢和肌肉萎缩[39, 48], 鳃的CRG主要参与离子调控[49]。
除了基因表达水平变化以外, 前体RNA发生低温诱导的可变剪接, 产生不同的可变剪接体(图 2), 也是鱼类低温应激反应的重要组分[41]。在低温条件下, 很多基因发生了可变剪接, 但是总的转录表达水平却保持不变[41, 50, 51]。RNA-seq在检测基因表达丰度的同时, 还能鉴定可变剪接和可变启动子使用等转录调控事件。通过对罗非鱼的42个RNA-seq数据集进行分析, 在脑和心脏中分别鉴定了483和208个低温调控的可变剪接事件[50]。对底鳉(Fundulus heteroclitus)、三刺鱼(Gasterosteus aculeatus)和斑马鱼的骨骼肌进行研究发现, 不同种类中发生低温诱导可变剪接的基因数目为426—866, 说明在低温适应过程中大量的基因发生了可变剪接[51]。低温诱导的可变剪接在调控拟南芥(Arabidopsis thaliana)的抗冻能力形成中具有重要作用[52], 但在调控鱼类抗寒中的作用和机制还不清楚。
研究鱼类CRG的表达调控对于解析其抗寒能力建成的分子机制具有重要的意义。转录因子、miRNA和lncRNA是主要的转录和转录后调控因子。转录因子AP-1的结合元件在斑马鱼低温诱导基因的上游序列中显著富集, 转录因子Jun与Bcl6的复合体通过与AP-1元件相结合调控下游基因的表达[44]。研究者们应用small RNA-seq技术鉴定了鱼类中受低温调控的miRNA及其靶基因。在斑马鱼仔鱼中发现dre-mir-29b调控低温诱导基因per2的表达[53]。在鲤肝脏中发现, 高温和低温刺激调控相同的miRNA, 但是作用的方向相反; 这些差异表达的miRNA主要参与调控糖皮质激素代谢和胰岛素信号通路[54]。在罗非鱼的肾脏中发现miR-29a/122能调控scd基因的表达[55]。在斑马鱼ZF4细胞中发现低温响应miRNA的靶基因主要参与磷酸化调控、细胞连接和细胞内信号传导等生物学过程, 使用这些miRNA的抑制剂或类似物处理ZF4细胞, 可增强细胞的低温耐受能力[56]。将致死低温暴露后的大菱鲆(Scophthalmus maximus) 分为耐受组和敏感组, 同时检测基因和miRNA表达, 鉴定了与抗寒能力相关的CRG和miRNA[57]。关于lncRNA调控鱼类低温应激反应的研究还很少。在斑马鱼ZF4细胞中鉴定了低温调控的lncRNA, 这些lncRNA可能参与调控电子转移、细胞黏附和氧化还原等生物学过程[58]。
2.4 低温应激反应的蛋白质组和代谢组研究
在低温条件下, 细胞优先翻译包含特定序列的mRNA分子, 从而调整细胞的蛋白质组成; 低温还诱导蛋白质的磷酸化和乙酰化等翻译后修饰, 使其化学性质发生改变, 更有利于在低温下发挥功能(图 2)。基于质谱分析的蛋白质组学技术极大地促进了低温调控的蛋白质及蛋白质翻译后修饰的鉴定。从虾虎鱼(Gillichthys mirabilis)的心脏中鉴定到37个受温度影响的蛋白质, 这些蛋白质主要参与能量代谢、线粒体调控和细胞骨架组织等生物学过程; 在9℃低温适应后, 虾虎鱼心脏肌酐激酶的表达丰度显著上升, 说明磷酸肌酐能量系统在低温条件下对心脏的功能具有重要作用[59]。在暗纹东方鲀(Takifugu fasciatus)的肝脏中发现了160个受低温调控的蛋白质, 低温诱导的RNA结合蛋白(CIRBP)、热激蛋白90(HSP90)和谷胱甘肽S-转移酶(GST)是代表性的低温诱导蛋白; 这些蛋白质富集的生物学过程包括氧化应激、线粒体酶和信号传导等[60]。对金头鲷的肝脏进行的定量蛋白质组研究发现, 低温调控的蛋白主要参与分解代谢[61]。从鲤的血浆中鉴定了22个低温诱导的蛋白, 这些蛋白质主要参与脂肪代谢和应激反应[62]。定量磷酸化蛋白质组研究从斑马鱼ZF4细胞中鉴定到702个低温条件下发生差异磷酸化修饰的位点, 分布于510个蛋白质; 这些差异磷酸化蛋白与蛋白质定位、细胞内转运和基因转录调控等生物学过程相关; 细胞外信号调节激酶(ERK1/2)是低温适应过程中调控蛋白质磷酸化的关键激酶[30]。在斑马鱼仔鱼上的研究也证明, 低温刺激可激活ERK1/2和p38 MAPK(Mitogen-activated protein kinase, 丝裂原活化蛋白激酶), 抑制其活性可显著降低仔鱼抗寒能力[63]。蛋白质组研究结果为认识鱼类抗寒能力建成的分子机制提供了数据支撑, 也为鱼类低温信号传导机制研究提供了重要线索。
转录组和蛋白质组研究可揭示应激状态下基因表达产物的结构和丰度等特征, 指示细胞代谢和生理状态的变化趋势, 而代谢组研究则检测细胞和组织中各种代谢物的含量。代谢物是细胞生命过程的终产物, 代谢物水平被认为是细胞对环境变化所产生最终反应[64]。对金头鲷的研究发现, 在降温过程中(28d内将温度从18℃降至11℃)肝脏的糖原、葡萄糖和ATP/ADP含量显著降低; 在低温维持过程中(11℃, 28d), 肝脏葡萄糖和ATP/ADP的含量回升; 而在恢复过程中(28d内将温度从11℃升高到18℃)肝脏糖原和葡萄糖含量进一步升高[65]。对许氏平鲉(Sebastes schlegelii)血液的研究发现, 急性低温刺激能引起脂肪酸代谢通路中相关代谢物水平的变化[66]。从大黄鱼的肝脏中鉴定了受低温和饥饿影响的代谢物, 这些代谢物主要与谷胱甘肽(GSH)代谢和不饱和脂肪酸合成等代谢通路有关[67]。对七彩神仙鱼(Symphysodon aequifasciatus)的鳃进行了代谢组研究, 发现慢性低温处理能激活抗氧化系统和GSH代谢[68]。以上研究结果表明, 能量代谢和抗氧化应激反应对鱼类抗寒能力的建成具有重要影响。
2.5 低温应激反应的“多组学”整合分析
机体和细胞的抗寒能力由不同类型的生物分子通过相互作用形成的网络共同决定。因此, 进行“多组学”的联合分析能有效地获得与抗寒相关的效应基因, 更全面地解析鱼类低温适应和抗寒能力形成的分子调控机制。在对暗纹东方鲀的肝脏进行的“多组学”(转录组、蛋白质组和代谢组)分析中, 发现了36个共表达的差异表达基因(DEG)-差异表达蛋白(DEP)对, 19个变化方向相反的DEG-DEP对, 及40个差异表达代谢物(DEM); 其中17个DEM和14个共表达的DEG-DEP对, 都参与脂肪酸代谢和膜转运等生物学过程[47]。
2.6 表观遗传修饰对鱼类抗寒能力形成的作用
低温可诱导组蛋白发生乙酰化等翻译后修饰, 基因组DNA发生甲基化和去甲基化等修饰(图 2)。大量研究关注鱼类的“热印记(Thermal imprint)[69]”, 即发育的早期阶段经历的温度环境对幼鱼或成鱼阶段面临温度胁迫时产生反应的影响。斑马鱼胚胎期的培养温度会影响成鱼在低温条件下的游泳能力、不同类型肌纤维的组成和肌肉组织对低温刺激的转录反应[70]。金头鲷胚胎和仔鱼阶段经历的“热印记”影响成鱼对低温胁迫的反应; 在低温处理后, 金头鲷血浆皮质醇、Na+、K+和葡萄糖水平, 及骨骼代谢均受早期发育阶段培养温度的影响[69]。胚胎期的培养温度还影响塞内加尔鳎(Solea senegalensis)和大西洋鳕(Gadus morhua)幼鱼不同组织中miRNA的表达[71, 72]。
这种“热印记”的实质是个体的“热经历”使其基因组的相关位点发生了可以长期持续的表观遗传修饰。在三刺鱼中研究了胚胎期的培养温度和成鱼期的适应温度对肌肉DNA甲基化和基因表达的影响, 发现irs2b、klhl38b、gadd45ga和slc3a2a是对温度刺激产生反应的代表性基因; 上调表达基因富集的生物学过程主要有细胞分裂、mRNA剪接和蛋白质降解, 下调表达基因富集的生物学过程主要包括细胞外基质组织和细胞黏附[73]。同时, 该研究还比较了差异表达基因和差异甲基化位点, 发现了324个低温或高温适应后与差异甲基化相关联的差异表达基因[73, 74]。该研究没有发现与发育阶段 “热印记”造成的差异表达基因相关联的DNA甲基化位点, 可能“热印记”的效应主要由其他类型的表观遗传修饰(如组蛋白乙酰化等)决定[73]。
2.7 鱼类抗寒相关基因的功能研究
虽然通过组学研究已经鉴定了大量的CRG, 但对这些基因在调控鱼类抗寒性状中的功能却知之甚少。目前, 已有研究者应用转基因、转座子介导的基因捕获和CRISPR/Cas9技术, 建立了转基因、基因插入失活和基因敲除的斑马鱼品系, 来研究目的基因在鱼类抗寒中的功能。例如通过研制转基因鱼品系, 在斑马鱼肌肉中表达鲤的Ⅲ型肌酐激酶(M3CK), 显著增强了其抗寒能力[75]。对斑马鱼grinaa基因转座子插入失活突变体的研究发现, Grinaa能减轻低温诱导的内质网应激和细胞凋亡, 从而提高鱼体的抗寒能力[76]。另外, 还应用CRISPR/Cas9技术获得了敲除自噬相关基因atg12和脂肪代谢相关基因cpt1b的斑马鱼品系, 发现这2个基因的突变都能降低斑马鱼的抗寒能力[77]。这些转基因和基因突变的斑马鱼品系对研究目的基因在调控鱼类抗寒中的功能和作用机制具有重要的意义。
3. 其他因子对鱼类抗寒能力的影响
3.1 低氧
在自然条件下, 由于栖息地的环境复杂多变, 鱼类往往会同时暴露于不同的环境刺激。对变温脊椎动物来说, 暴露于不同类型的刺激, 都会造成组织缺氧和氧化应激[6]。温度和低氧是对鱼类生理功能具有重要影响, 且能相互作用的两种环境刺激因子[78]。鱼类在长期的演化过程中形成了对低氧和低温等环境胁迫的适应能力。对斑马鱼仔鱼的研究发现, 低氧适应(5%氧气)能显著增强个体对致死低温(12℃)的耐受能力, 而低温适应(18℃)却使个体对致死低氧处理(2.5%氧气)更加敏感[79]; RNA-seq分析结果表明, 低温和低氧共诱导的基因主要参与氧化还原过程、氧气转运、造血、血红蛋白合成和细胞离子平衡[79]。低温和低氧胁迫都能诱导细胞和组织产生ROS[80, 81]。急性低温暴露诱导斑马鱼肝脏产生氧化应激, 投喂含α-硫辛酸或GSH等还原剂的饲料可以显著降低低温引起的氧化应激, 进而减轻组织损伤, 增强抗寒能力; 而H2O2处理诱导氧化应激则能降低斑马鱼ZFL细胞的耐寒能力[82]。
3.2 盐度
盐度是影响鱼类生理的一个重要环境因子。降河洄游鱼类会同时遭受高盐度和低温刺激[83]。水体盐度对遮目鱼(Chanos chanos)的抗寒能力具有明显的影响, 在海水中适应的个体与在淡水中适应的个体相比, 抗寒能力显著增强[84]。低盐度刺激可能通过影响低温条件下遮目鱼肝脏糖原的分解代谢[85]、ATP合成[86]和鳃的离子调控[84], 来影响其抗寒能力。对刀鲚(Coilia nasus)同时进行高盐度和低温刺激, 发现神经递质、受体和调控蛋白可能在洄游中起着重要的调控作用[83]。
另外, 一些低温响应基因能被多种环境刺激诱导表达。如斑马鱼nr1d4a和nr1d4b基因能被重金属、缺氧和高盐度处理诱导表达[87]。这些能被多种环境刺激的诱导表达的基因可能代表细胞应激保护机制的核心模块, 低氧和盐度等环境刺激因子可能通过调控这些基因的表达影响鱼类的低温应激反应和抗寒能力。
3.3 饥饿和营养
低温胁迫会降低鱼的摄食率, 引起饥饿[77]。虽然长期的饥饿会导致能量耗竭, 但是一定水平的饥饿不仅可以优化营养物质代谢, 还可以通过增强自噬减轻细胞损伤[88]。研究发现, 饥饿48h能显著增强斑马鱼的抗寒能力; 脂肪分解代谢和自噬的激活在禁食诱导的抗寒效应中起着关键作用; 另外, 抑制mTOR信号通路可以起到与禁食一样的抗寒效果, 而激活mTOR信号通路则能减弱抗寒效应[77]; 根据该研究的结果, 养殖生产中适当禁食可以作为提高鱼类抗寒能力的策略[77]。饲料的营养成分, 如脂肪的来源也能影响鱼类的抗寒能力。尼罗罗非鱼(Oreochromis niloticus)摄食添加玉米油或玉米油与鱼油混合物(1﹕1)的饲料时, 与添加鱼油或可可油的饲料相比, 抗寒能力显著增强[89]。该研究结果说明可以通过营养调控来增强罗非鱼的抗寒能力。
4. 遗传多样性与鱼类的抗寒性状
鱼类等水产动物大多数都处于家养驯化的早期阶段, 种内遗传多样性丰富, 可以通过遗传筛选获得显著的性状改良效应[90]。很多养殖鱼类有众多地方品种和选育品系, 不同的品种在体色、鳞片和体型等形态特征和应对环境胁迫的能力方面具有明显差异。例如, 对于我国主要养殖鱼类之一的鲤而言, 北方的黑龙江野鲤和人工培育的松浦镜鲤都具有很强的耐寒能力, 可以在冬季长期冰封的池塘中生存, 但鲤的近缘种柏氏鲤(Cyprinus pellegrini Tchang)却对低温非常敏感[91]。研究者们曾经尝试应用RAPD(随机扩增多态性DNA)和SSR(简单重复序列)等标记定位鲤耐寒相关QTL位点[91, 92]; 但由于检测通量小, 标记数目不足, 遗传图谱不完全, 鉴定到的标记难以定位到染色体上[91]。因此, 迄今尚未克隆到决定鲤耐寒能力的关键基因或调控元件。
最近的研究发现, hsp70和hmgb1基因序列中的单核苷酸多态性位点(SNP)与牙鲆(Paralichthys olivaceus)的抗寒能力显著相关[93], 其中hmgb1是多种鱼类中受到低温刺激时高水平上调表达的标记基因[39, 40]。对底鳉肌肉组织的研究结果表明, 线粒体的基因型对低温应激条件下的基因表达也有一定影响[94]。研究核基因组和线粒体基因组遗传多样性与鱼类抗寒能力的关系有望开发抗寒相关的分子标记, 促进鱼类的抗寒育种。
5. 展望
在应用各种组学技术开展的鱼类低温应激反应研究中, 发现了大量的低温响应基因, 但是大部分基因的功能都还不清楚。在斑马鱼等模式鱼上, 应用基因工程和基因编辑技术研制转基因、基因敲除和基因定点编辑的品系, 可以为系统解析目的基因的作用机制提供研究模型。在经济鱼类中, 可通过全基因组关联分析(GWAS)和数量性状位点(QTL)定位等技术鉴定影响抗寒能力的关键变异位点和分子模块, 开发分子标记, 开展主要养殖品种的分子标记辅助抗寒育种。另外, 根据模式鱼中抗寒相关基因的作用机制及关键位点的精细定位等研究结果, 对经济鱼类相应的基因及其调控序列进行定点编辑, 有望使其耐寒能力显著增强。
-
图 2 鱼类低温应激反应的遗传调控
H. 组蛋白; A. 乙酰化修饰; P. 磷酸化修饰; 可变剪接. 绿色的点表示mRNA的5′端帽子结构, 线条中蓝色部分表示内含子, 红色表示外显子; 线条的粗细表示RNA分子的丰度; 翻译调控. 蓝色线条的粗细表示肽链的翻译效率; 代谢物变化. 图形的数目表示不同代谢物的丰度
Figure 2. Genetic regulation underlying cold stress responses of fish
H. histone; A. acetylation; P. phosphorylation; Alternative splicing: the green dots stand for 5′ cap of mRNA molecules, the blue segment in the lines stands for intron and the red segment stands for exon, while thickness of the lines denotes abundance of the RNA molecules; Translation regulation: thickness of the blue lines indicates translational efficiency of the peptides; Change in metabolites: numbers of the geometric figures represent abundance of different metabolites
-
[1] Donaldson M R, Cooke S J, Patterson D A, et al. Cold shock and fish [J]. Journal of Fish Biology, 2008, 73(7): 1491-1530. doi: 10.1111/j.1095-8649.2008.02061.x
[2] Hofmann G E, Todgham A E. Living in the now: physiological mechanisms to tolerate a rapidly changing environment [J]. Annual Review of Physiology, 2010, 72(1): 127-145. doi: 10.1146/annurev-physiol-021909-135900
[3] Xu D D, You Q C, Chi C F, et al. Transcriptional response to low temperature in the yellow drum (Nibea albiflora) and identification of genes related to cold stress [J]. Comparative Biochemistry and Physiology-Part D:Genomics and Proteomics, 2018(28): 80-89.
[4] Sun Z, Tan X, Xu M, et al. Liver transcriptome analysis and de novo annotation of the orange-spotted groupers (Epinephelus coioides) under cold stress [J]. Comparative Biochemistry and Physiology-Part D:Genomics and Proteomics, 2019(29): 264-273.
[5] Mininni A N, Milan M, Ferraresso S, et al. Liver transcriptome analysis in gilthead sea bream upon exposure to low temperature [J]. BMC Genomics, 2014(15): 765.
[6] Kassahn K S, Crozier R H, Portner H O, et al. Animal performance and stress: responses and tolerance limits at different levels of biological organisation [J]. Biological Reviews, 2009, 84(2): 277-292. doi: 10.1111/j.1469-185X.2008.00073.x
[7] Somero G N, Hochachka P W. Biochemical adaptation to environment [J]. American Zoologist, 1971, 11(1): 159-167. doi: 10.1093/icb/11.1.159
[8] Zhang Y, Burkhardt D H, Rouskin S, et al. A stress response that monitors and regulates mRNA structure is central to cold shock adaptation [J]. Molecular Cell, 2018, 70(2): 274-286. doi: 10.1016/j.molcel.2018.02.035
[9] He J, Qiang J, Yang H, et al. Changes in the fatty acid composition and regulation of antioxidant enzymes and physiology of juvenile genetically improved farmed tilapia Oreochromis niloticus (L.), subjected to short-term low temperature stress [J]. Journal of Thermal Biology, 2015(53): 90-97.
[10] Somero G N. The cellular stress response and temperature: Function, regulation, and evolution [J]. Journal of Experimental Zoology Part a-Ecological and Integrative Physiology, 2020, 333(6): 379-397. doi: 10.1002/jez.2344
[11] Los D A, Murata N. Membrane fluidity and its roles in the perception of environmental signals [J]. Biochim Biophys Acta, 2004, 1666(1-2): 142-157. doi: 10.1016/j.bbamem.2004.08.002
[12] 邹曙明, 楼允东, 沈俊宝, 等. 鱼类低温适应机制及抗寒育种 [J]. 上海水产大学学报, 1998, 7(3): 231-237. Zou S M, Lou Y D, Shen J B, et al. The mechanisms of cold acclimatization and cold-tolerance breeding in fish [J]. Journal of Shanghai Fisheries University, 1998, 7(3): 231-237.
[13] Eckerle L G, Lucassen M, Hirse T, et al. Cold induced changes of adenosine levels in common eelpout (Zoarces viviparus): a role in modulating cytochrome c oxidase expression [J]. Journal of Experimental Biology, 2008, 211(8): 1262-1269. doi: 10.1242/jeb.013474
[14] Ou J X, Ball J M, Luan Y Z, et al. iPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications [J]. Cell, 2018, 173(4): 851-863. doi: 10.1016/j.cell.2018.03.010
[15] Hattori K, Ishikawa H, Sakauchi C, et al. Cold stress-induced ferroptosis involves the ASK1-p38 pathway [J]. EMBO Reports, 2017, 18(11): 2067-2078. doi: 10.15252/embr.201744228
[16] van den Burg E H, Peeters R R, Verhoye M, et al. Brain responses to ambient temperature fluctuations in fish: reduction of blood volume and initiation of a whole-body stress response [J]. Journal of Neurophysiology, 2005, 93(5): 2849-2855. doi: 10.1152/jn.01113.2004
[17] Black M C, Millsap D S, Mccarthy J F. Effects of acute temperature-change on respiration and toxicant uptake by rainbow-trout, salmo-gairdneri (Richardson) [J]. Physiological Zoology, 1991, 64(1): 145-168. doi: 10.1086/physzool.64.1.30158517
[18] Zhou T, Gui L, Liu M, et al. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus [J]. Fish & Shellfish Immunology, 2019(84): 1145-1156.
[19] Abram Q H, Dixon B, Katzenback B A. Impacts of low temperature on the teleost immune system [J]. Biology-Basel, 2017, 6(4): 39.
[20] Johnston I A, Dunn J. Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish [J]. Symposia of the Society for Experimental Biology, 1987(41): 67-93.
[21] Hurst T P, Conover D O. Winter mortality of young-of-the-year Hudson River striped bass (Morone saxatilis): size-dependent patterns and effects on recruitment [J]. Canadian Journal of Fisheries and Aquatic Sciences, 1998, 55(5): 1122-1130. doi: 10.1139/f98-017
[22] Hurst T P. Causes and consequences of winter mortality in fishes [J]. Journal of Fish Biology, 2007, 71(2): 315-345. doi: 10.1111/j.1095-8649.2007.01596.x
[23] Kortmann J, Narberhaus F. Bacterial RNA thermometers: molecular zippers and switches [J]. Nature Reviews Microbiology, 2012, 10(4): 255-265. doi: 10.1038/nrmicro2730
[24] Teets N M, Yi S X, Lee R E, et al. Calcium signaling mediates cold sensing in insect tissues [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(22): 9154-9159. doi: 10.1073/pnas.1306705110
[25] Peier A M, Moqrich A, Hergarden A C, et al. A TRP channel that senses cold stimuli and menthol [J]. Cell, 2002, 108(5): 705-715. doi: 10.1016/S0092-8674(02)00652-9
[26] Madden C J, Morrison S F. Central nervous system circuits that control body temperature [J]. Neuroscience Letters, 2019(696): 225-232. doi: 10.1016/j.neulet.2018.11.027
[27] Bautista D M, Siemens J, Glazer J M, et al. The menthol receptor TRPM8 is the principal detector of environmental cold [J]. Nature, 2007, 448(7150): 204-208. doi: 10.1038/nature05910
[28] Feketa V V, Nikolaev Y A, Merriman D K, et al. CNGA3 acts as a cold sensor in hypothalamic neurons [J]. Elife, 2020(9): e55370. doi: 10.7554/eLife.55370
[29] Gong J K, Liu J Z, Ronan E A, et al. A cold-sensing receptor encoded by a glutamate receptor gene [J]. Cell, 2019, 178(6): 1375-1386. doi: 10.1016/j.cell.2019.07.034
[30] Yan J J, Long Y, Zhou T, et al. Dynamic phosphoproteome profiling of zebrafish embryonic fibroblasts during cold acclimation [J]. Proteomics, 2020, 20(2): e1900257. doi: 10.1002/pmic.201900257
[31] Barton B A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids [J]. Integrative and Comparative Biology, 2002, 42(3): 517-525. doi: 10.1093/icb/42.3.517
[32] Mommsen T P, Vijayan M M, Moon T W. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation [J]. Reviews in Fish Biology and Fisheries, 1999, 9(3): 211-268. doi: 10.1023/A:1008924418720
[33] Chen W H, Sun L T, Tsai C L, et al. Cold-stress induced the modulation of catecholamines, cortisol, immunoglobulin M, and leukocyte phagocytosis in tilapia [J]. General and Comparative Endocrinology, 2002, 126(1): 90-100. doi: 10.1006/gcen.2001.7772
[34] Tanck M W T, Booms G H R, Eding E H, et al. Cold shocks: a stressor for common carp [J]. Journal of Fish Biology, 2000, 57(4): 881-894. doi: 10.1111/j.1095-8649.2000.tb02199.x
[35] Faught E, Vijayan M M. Mechanisms of cortisol action in fish hepatocytes [J]. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology, 2016(199): 136-145.
[36] Little A G, Kunisue T, Kannan K, et al. Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio) [J]. BMC Biology, 2013(11): 26. doi: 10.1186/1741-7007-11-26
[37] Little A G, Seebacher F. Thyroid hormone regulates muscle function during cold acclimation in zebrafish (Danio rerio) [J]. Journal of Experimental Biology, 2013, 216(18): 3514-3521. doi: 10.1242/jeb.089136
[38] Little A G, Seebacher F. Thyroid hormone regulates cardiac performance during cold acclimation in zebrafish (Danio rerio) [J]. Journal of Experimental Biology, 2014, 217(5): 718-725.
[39] Gracey A Y, Fraser E J, Li W Z, et al. Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(48): 16970-16975. doi: 10.1073/pnas.0403627101
[40] Long Y, Li L, Li Q, et al. Transcriptomic characterization of temperature stress responses in larval zebrafish [J]. PLoS One, 2012, 7(5): e37209. doi: 10.1371/journal.pone.0037209
[41] Long Y, Song G, Yan J, et al. Transcriptomic characterization of cold acclimation in larval zebrafish [J]. BMC Genomics, 2013(14): 612. doi: 10.1186/1471-2164-14-612
[42] 李林春, 李青, 龙勇, 等. 温度刺激对斑马鱼仔鱼基因转录表达的影响 [J]. 水生生物学报, 2012, 36(5): 882-891. Li L C, Li Q, Long Y, et al. Microarray analysis of temperature stress effects on transcriptional expression in zebrafish larvae [J]. Acta Hydrobiologica Sinica, 2012, 36(5): 882-891.
[43] Liang L Q, Chang Y M, He X L, et al. Transcriptome analysis to identify cold-responsive genes in Amur carp (Cyprinus carpio haematopterus) [J]. PLoS One, 2015, 10(6): e0130526. doi: 10.1371/journal.pone.0130526
[44] Hu P, Liu M, Zhang D, et al. Global identification of the genetic networks and cis-regulatory elements of the cold response in zebrafish [J]. Nucleic Acids Research, 2015, 43(19): 9198-9213. doi: 10.1093/nar/gkv780
[45] Long Y, Li X X, Li F Y, et al. Transcriptional programs underlying cold acclimation of common carp (Cyprinus carpio L.) [J]. Frontiers in Genetics, 2020(11): 556418.
[46] Hu P, Liu M L, Liu Y M, et al. Transcriptome comparison reveals a genetic network regulating the lower temperature limit in fish [J]. Scientific Reports, 2016(6): 28952. doi: 10.1038/srep28952
[47] Wen X, Hu Y D, Zhang X Y, et al. Integrated application of multi-omics provides insights into cold stress responses in pufferfish Takifugu fasciatus [J]. BMC Genomics, 2019, 20(1): 563. doi: 10.1186/s12864-019-5915-7
[48] Ikeda D, Koyama H, Mizusawa N, et al. Global gene expression analysis of the muscle tissues of medaka acclimated to low and high environmental temperatures [J]. Comparative Biochemistry and Physiology D-Genomics & Proteomics, 2017(24): 19-28.
[49] Chou M Y, Hsiao C D, Chen S C, et al. Effects of hypothermia on gene expression in zebrafish gills: upregulation in differentiation and function of ionocytes as compensatory responses [J]. Journal of Experimental Biology, 2008, 211(19): 3077-3084. doi: 10.1242/jeb.019950
[50] Li B J, Zhu Z X, Qin H, et al. Genome-wide characterization of alternative splicing events and their responses to cold stress in tilapia [J]. Frontiers in Genetics, 2020(11): 244. doi: 10.3389/fgene.2020.00244
[51] Healy T M, Schulte P M. Patterns of alternative splicing in response to cold acclimation in fish [J]. Journal of Experimental Biology, 2019, 222(5): jeb193516.
[52] Calixto C P G, Guo W B, James A B, et al. Rapid and dynamic alternative splicing impacts the arabidopsis cold response transcriptome [J]. Plant Cell, 2018, 30(7): 1424-1444. doi: 10.1105/tpc.18.00177
[53] Hung I C, Hsiao Y C, Sun H S, et al. microRNAs regulate gene plasticity during cold shock in zebrafish larvae [J]. BMC Genomics, 2016, 17(1): 922. doi: 10.1186/s12864-016-3239-4
[54] Sun J L, Zhao L L, Wu H, et al. Analysis of miRNA-seq in the liver of common carp (Cyprinus carpio L.) in response to different environmental temperatures [J]. Functional & Integrative Genomics, 2019, 19(2): 265-280.
[55] Qiang J, Cui Y T, Tao F Y, et al. Physiological response and microRNA expression profiles in head kidney of genetically improved farmed tilapia (GIFT, Oreochromis niloticus) exposed to acute cold stress [J]. Scientific Reports, 2018, 8(1): 172. doi: 10.1038/s41598-017-18512-6
[56] Ji X, Jiang P, Luo J, et al. Identification and characterization of miRNAs involved in cold acclimation of zebrafish ZF4 cells [J]. PLoS One, 2020, 15(1): e0226905. doi: 10.1371/journal.pone.0226905
[57] Nie M M, Tan X G, Lu Y L, et al. Network of microRNA-transcriptional factor-mRNA in cold response of turbot Scophthalmus maximus [J]. Fish Physiology and Biochemistry, 2019, 45(2): 583-597. doi: 10.1007/s10695-019-00611-y
[58] Jiang P, Hou Y, Fu W, et al. Characterization of lncRNAs involved in cold acclimation of zebrafish ZF4 cells [J]. PLoS One, 2018, 13(4): e0195468. doi: 10.1371/journal.pone.0195468
[59] Jayasundara N, Tomanek L, Dowd W W, et al. Proteomic analysis of cardiac response to thermal acclimation in the eurythermal goby fish Gillichthys mirabilis [J]. Journal of Experimental Biology, 2015, 218(9): 1359-1372. doi: 10.1242/jeb.118760
[60] Wen X, Zhang X Y, Hu Y D, et al. iTRAQ-based quantitative proteomic analysis of Takifugu fasciatus liver in response to low-temperature stress [J]. Journal of Proteomics, 2019(201): 27-36. doi: 10.1016/j.jprot.2019.04.004
[61] Ghisaura S, Pagnozzi D, Melis R, et al. Liver proteomics of gilthead sea bream (Sparus aurata) exposed to cold stress [J]. Journal of Thermal Biology, 2019(82): 234-241. doi: 10.1016/j.jtherbio.2019.04.005
[62] Dietrich M A, Hliwa P, Adamek M, et al. Acclimation to cold and warm temperatures is associated with differential expression of male carp blood proteins involved in acute phase and stress responses, and lipid metabolism [J]. Fish & Shellfish Immunology, 2018(76): 305-315.
[63] Ren J, Long Y, Liu R, et al. Characterization of biological pathways regulating acute cold resistance of zebrafish [J]. International Journal of Molecular Sciences, 2021, 22(6): 3028. doi: 10.3390/ijms22063028
[64] Fiehn O. Metabolomics - the link between genotypes and phenotypes [J]. Plant Molecular Biology, 2002, 48(1-2): 155-171.
[65] Melis R, Sanna R, Braca A, et al. Molecular details on gilthead sea bream (Sparus aurata) sensitivity to low water temperatures from H-1 NMR metabolomics [J]. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology, 2017(204): 129-136.
[66] Song M, Zhao J, Wen H S, et al. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii) [J]. PLoS One, 2019, 14(5): e0217133. doi: 10.1371/journal.pone.0217133
[67] Jiao S, Nie M M, Song H B, et al. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics [J]. Science of the Total Environment, 2020(715): 136940. doi: 10.1016/j.scitotenv.2020.136940
[68] Wen B, Jin S R, Chen Z Z, et al. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics [J]. Science of the Total Environment, 2018(640): 1372-1381.
[69] Mateus A P, Costa R, Gisbert E, et al. Thermal imprinting modifies bone homeostasis in cold-challenged sea bream (Sparus aurata) [J]. Journal of Experimental Biology, 2017, 220(19): 3442-3454.
[70] Scott G R, Johnston I A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(35): 14247-14252. doi: 10.1073/pnas.1205012109
[71] Campos C, Sundaram A Y M, Valente L M P, et al. Thermal plasticity of the miRNA transcriptome during Senegalese sole development [J]. BMC Genomics, 2014, 15(1): 525. doi: 10.1186/1471-2164-15-525
[72] Bizuayehu T T, Johansen S D, Puvanendran V, et al. Temperature during early development has long-term effects on microRNA expression in Atlantic cod [J]. BMC Genomics, 2015, 16(1): 305. doi: 10.1186/s12864-015-1503-7
[73] Metzger D C H, Schulte P M. Similarities in temperature-dependent gene expression plasticity across timescales in threespine stickleback (Gasterosteus aculeatus) [J]. Molecular Ecology, 2018, 27(10): 2381-2396. doi: 10.1111/mec.14591
[74] Metzger D C H, Schulte P M. Persistent and plastic effects of temperature on DNA methylation across the genome of threespine stickleback (Gasterosteus aculeatus) [J]. Proceedings of the Royal Society B-Biological Sciences, 2017, 284(1864): 20171667. doi: 10.1098/rspb.2017.1667
[75] Wang Q, Tan X G, Jiao S, et al. Analyzing cold tolerance mechanism in transgenic zebrafish (Danio rerio) [J]. PLoS One, 2014, 9(7): e102492. doi: 10.1371/journal.pone.0102492
[76] Chen K, Li X X, Song G L, et al. Deficiency in the membrane protein Tmbim3a/Grinaa initiates cold-induced ER stress and cell death by activating an intrinsic apoptotic pathway in zebrafish [J]. Journal of Biological Chemistry, 2019, 294(30): 11445-11457. doi: 10.1074/jbc.RA119.007813
[77] Lu D L, Ma Q, Wang J, et al. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy [J]. Journal of Physiology-London, 2019, 597(6): 1585-1603. doi: 10.1113/JP277091
[78] McBryan T L, Anttila K, Healy T M, et al. Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change [J]. Integrative and Comparative Biology, 2013, 53(4): 648-659. doi: 10.1093/icb/ict066
[79] Long Y, Yan J J, Song G L, et al. Transcriptional events co-regulated by hypoxia and cold stresses in zebrafish larvae [J]. BMC Genomics, 2015, 16(1): 385. doi: 10.1186/s12864-015-1560-y
[80] Clanton T L. Hypoxia-induced reactive oxygen species formation in skeletal muscle [J]. Journal of Applied Physiology, 2007, 102(6): 2379-2388. doi: 10.1152/japplphysiol.01298.2006
[81] Tseng Y C, Chen R D, Lucassen M, et al. Exploring uncoupling proteins and antioxidant mechanisms under acute cold exposure in brains of fish [J]. PLoS One, 2011, 6(3): e18180. doi: 10.1371/journal.pone.0018180
[82] Lu D L, Ma Q, Sun S X, et al. Reduced oxidative stress increases acute cold stress tolerance in zebrafish [J]. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology, 2019(235): 166-173.
[83] Wang M Y, Xu G C, Tang Y K, et al. Transcriptome analysis of the brain provides insights into the regulatory mechanism for Coilia nasus migration [J]. BMC Genomics, 2020, 21(1): 410. doi: 10.1186/s12864-020-06816-3
[84] Kang C K, Chen Y C, Chang C H, et al. Seawater-acclimation abates cold effects on Na+, K+-ATPase activity in gills of the juvenile milkfish, Chanos chanos [J]. Aquaculture, 2015(446): 67-73. doi: 10.1016/j.aquaculture.2015.04.022
[85] Chang C H, Huang J J, Yeh C Y, et al. Salinity effects on strategies of glycogen utilization in livers of eurhaline milkfish (Chanos chanos) under hypothermal stress [J]. Frontiers in Physiology, 2018(9): 81. doi: 10.3389/fphys.2018.00081
[86] Chang C H, Liu Z Z, Lee T H. Changes in hypothermal stress-induced hepatic mitochondrial metabolic patterns between fresh water- and seawater-acclimated milkfish, Chanos chanos [J]. Scientific Reports, 2019, 9(1): 18502. doi: 10.1038/s41598-019-55055-4
[87] 杨玲, 严军军, 龙勇, 等. 斑马鱼nr1d4a和nr1d4b基因的表达及其对不同环境刺激的响应 [J]. 水生生物学报, 2014, 38(1): 100-107. Yang L, Yan J J, Long Y, et al. Expression patterns of zebrafish nr1d4a and nr1d4b and their transcriptional responses to different environmental stresses [J]. Acta Hydrobiologica Sinica, 2014, 38(1): 100-107.
[88] Libert S, Guarente L. Metabolic and neuropsychiatric effects of calorie restriction and sirtuins [J]. Annual Review of Physiology, 2013(75): 669-684. doi: 10.1146/annurev-physiol-030212-183800
[89] Abdel-Ghany H M, El-Sayed A F M, Ezzat A A, et al. Dietary lipid sources affect cold tolerance of Nile tilapia (Oreochromis niloticus) [J]. Journal of Thermal Biology, 2019(79): 50-55. doi: 10.1016/j.jtherbio.2018.11.009
[90] Houston R D, Bean T P, Macqueen D J, et al. Harnessing genomics to fast-track genetic improvement in aquaculture [J]. Nature Reviews Genetics, 2020, 21(7): 389-409. doi: 10.1038/s41576-020-0227-y
[91] Zheng X, Kuang Y, Zhang X, et al. A genetic linkage map and comparative genome analysis of common carp (Cyprinus carpio L.) using microsatellites and SNPs [J]. Molecular Genetics and Genomics, 2011, 286(3-4): 261-277.
[92] 潘贤, 梁利群, 雷清泉. 筛选与鲤鱼抗寒性状相关的微卫星分子标记 [J]. 哈尔滨工业大学学报, 2008, 40(6): 915-918. doi: 10.3321/j.issn:0367-6234.2008.06.017 Pan X, Liang L Q, Lei Q Q. Selection of microsatellite molecular markers associated with the character of cold tolerance of common carp [J]. Journal of Harbin Institute of Technology, 2008, 40(6): 915-918. doi: 10.3321/j.issn:0367-6234.2008.06.017
[93] Nie M M, Hu J W, Lu Y L, et al. Cold effect analysis and screening of SNPs associated with cold-tolerance in the olive flounder Paralichthys olivaceus [J]. Journal of Applied Ichthyology, 2019, 35(4): 924-932.
[94] Healy T M, Bryant H J, Schulte P M. Mitochondrial genotype and phenotypic plasticity of gene expression in response to cold acclimation in killifish [J]. Molecular Ecology, 2017, 26(3): 814-830. doi: 10.1111/mec.13945
-
期刊类型引用(12)
1. 肖文杰,郭宝英,赵超,薛月光,刘长琳,姜达,司仲强. 高温胁迫对膨腹海马幼苗存活率及抗氧化酶活性的影响. 动物学杂志. 2025(01): 59-68 .  百度学术
百度学术
2. 秦俊杰,梁化亮,黄东宇,任鸣春. 多糖类饲料添加剂在鱼类饲料中应用的研究进展. 饲料工业. 2025(05): 25-32 .  百度学术
百度学术
3. 叶君玉,李衍红,李以玲,高阳,夏斌,崔正国,曲克明. 铁基金属有机骨架对中华青鳉毒性作用的持续性与恢复性. 生态毒理学报. 2025(02): 152-160 .  百度学术
百度学术
4. 金鲟,刘炳舰,竺奇慧,张万昌,李完波,王志勇,高天翔,徐冬冬. 黄姑鱼低温耐受性的全基因组关联分析. 水产学报. 2025(06): 44-55 .  百度学术
百度学术
5. 胡晓娜,吴兴兵,朱永久,朱挺兵,李学梅,杨德国. 圆口铜鱼呼吸代谢的温度响应特征研究. 淡水渔业. 2024(01): 86-92 .  百度学术
百度学术
6. 朱琳,祝璟琳,邹芝英,李大宇,肖炜,喻杰,陈炳霖,杨弘. 五品系尼罗罗非鱼对自然慢性低温胁迫的生理响应. 淡水渔业. 2024(03): 42-51 .  百度学术
百度学术
7. 高阳,王超宇,刘鑫,梁夏颖,赵哲,史燕. 暗纹东方鲀耐寒相关基因CIRBP、HMGB1和AFP-Ⅳ的分子特征和对低温胁迫的响应. 水生生物学报. 2023(05): 756-766 .  本站查看
本站查看
8. 李豫,黄建盛,陈有铭,温震威,欧光海,黄鉴鹏,蒋鑫涛,谢瑞涛,马骞,陈刚. 低温胁迫对军曹鱼幼鱼鳃组织抗氧化能力、细胞凋亡和组织结构的影响. 南方水产科学. 2023(03): 68-77 .  百度学术
百度学术
9. 唐家铭,蒋乐霞,张长峰,黄宝生,陈东杰,姜沛宏,赵如轩,胡超. 维生素E对鲫鱼生理生化、肌肉质构及风味的影响及其缓解降温胁迫作用研究. 食品与发酵工业. 2023(19): 226-234 .  百度学术
百度学术
10. 时昕晔. 寒冷环境下鱼类抗寒抗冻的细胞内在机制探讨. 湖北农业科学. 2022(11): 163-169 .  百度学术
百度学术
11. 卫明亮,张志伟,张志勇,林志杰,祝斐,贾超峰,孟乾,徐大凤,张曹进. 冷应激对黑鲷组织损伤及细胞凋亡基因表达的影响. 南方水产科学. 2022(05): 110-117 .  百度学术
百度学术
12. 曾霖,王永红,宋炜,谢正丽,张惠. 基于转录组解析铜驯化对低温胁迫下大黄鱼氧化损伤的影响. 中国水产科学. 2022(10): 1425-1436 .  百度学术
百度学术
其他类型引用(12)



 下载:
下载: